Rodolfo Pizarro ID
Head of Cardiology, Hospital Italiano de Buenos Aires. Master in Clinical
Effectiveness.
Ciudad Autónoma de Buenos Aires, Argentina.
Acta Gastroenterol Latinoam 2022;52(3):300-302
Received: 15/08/2022 / Accepted: 16/09/2022 / Published online 29/09/2022 / https://doi.org/10.52787/agl.v52i3.236
Non-inferiority clinical trials are conducted because the experimental treatment is not considered to be better than the standard treatment, but it offers additional advantages, such as: fewer side effects, better metabolic profile, easier administration, less controls and minor costs.
The nature of the research and the answers are different, as in this type of trials we ask whether the experimental treatment is at least as effective as the standard.
It can either be at least as effective as the standard (alternative hypothesis) or less effective than the standard (null hypothesis).
Considering the p-value in non-inferiority studies is important, since we must specify a priori what we consider to be at least as effective or non-inferior. For this, a minimum margin of non-inferiority must be defined.
Therefore, let us consider non-inferiority studies under the following hypothesis:
- Difference in experimental treatment - standard treatment > margin of non-inferiority (null hypothesis).
- Difference in experimental treatment - standard treatment < = margin of non-inferiority (alternative hypothesis), that is, the difference between the experimental treatment and the active control does not exceed the margin of non-inferiority.
The following question answers how to set the minimum non-inferiority margin:
1. Through previous studies that establish the lower limits of the standard intervention vs. placebo.
2. Establishing a previous percentage limit for the new treatment of 50 or 80% of the effect in the classic intervention.
Although what we discussed above is crucial for this type of study, the inferiority limit must be established a priori so that it does not lend itself to subsequent manipulations.
An example is the study of warfarin in reducing the incidence of embolic events compared to placebo with a relative risk of 0.38 (95% CI: 0.28-0.52).
First, we changed the risk category for the placebo (1/0.38) which would mean a RR: 2.63 (95% CI: 1.92-3.57), being able to consider the minimum margin of non-inferiority to be 1.92. However, in this case, a higher requirement was sought, with 50% of the effect of warfarin, and it was established at 1.46. This value was set as the non-inferiority limit.
That is to say the upper limit of the 95% CI of the effect of the new treatment, with respect to warfarin, could not exceed 1.46.
Another example is the ONTARGET study that was designed to demonstrate the non-inferiority of telmisartan (80 mg per day) vs ramipril (10 mg per day) with a combined endpoint of CV death, stroke, myocardial infarction, or hospitalization for pump failure.
To determine the margin of non-inferiority, the HOPE study was used, which evaluated ramipril versus placebo with a combined endpoint similar to ONTARGET.
In this study, the hazard ratio was in favor of ramipril, with a RR of 0.78 (95% CI 0.70-0.86); this was transformed to an excess risk for placebo (1/0.78) of 1.26, and a non-inferiority limit of 1.13 (50% effect of ramipril for telmisartan in the ONTARGET study) was established.
The results of this study determined that telmisartan compared to ramipril showed a RR of 1.01 (95% CI: 0.94-1.09) p < 0.0033, which is lower than the non-inferiority limit of 1.13 .
Telmisartan was considered non-inferior to ramipril, adding some advantages to consider: study discontinuation was higher for ramipril (7.2%) than for telmisartan (5.1%).
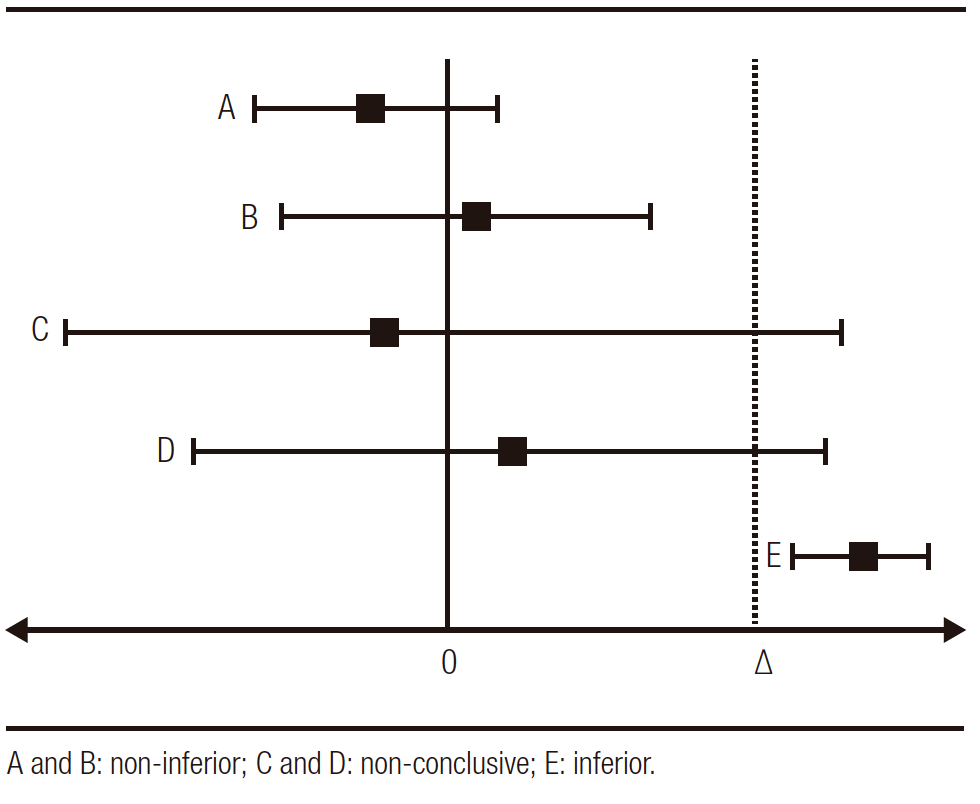
Graphically we can determine the non-inferiority studies in the following scheme of Figure 1 (∆: pre-established non-inferiority limit):
Figure 1. Possible non-inferiority results 
Next, we will cite another example, this time on topics related to gastroenterology, entitled: "A Randomized, Double-Blind, Active-Control, Noninferiority, Multicenter, Phase 4 Study to Evaluate the Efficacy and Safety of Esomeprazole/Sodium Bicarbonate 20/800 mg in Patients with Nonerosive Gastroesophageal Reflux Disease”.
Non-erosive reflux disease (NERD) is the most frequent phenotype (60-70%). The therapeutic goal is to improve symptoms with an on-demand strategy. That is, to give the patient the minimum dose with which he/she is asymptomatic. In this sense, it must be emphasized that first-generation proton pump inhibitors (PPIs) must be administered on an empty stomach and associated with a subsequent meal so as not to delay adequate absorption and, consequently, their bioavailability. To improve this situation, combined drugs (PPI+sodium bicarbonate) are currently available. Said formulation would make it possible to avoid the degradation of the drug due to the gastric pH, to have a faster absorption and, with it, a symptomatic improvement. In addition, the presence of bicarbonate is a stimulant of gastrin secretion since it lowers gastric pH; therefore, post-meal intake would not be a condition for the release of the hormone.
The aforementioned study evaluated the efficacy and safety of esomeprazole/sodium bicarbonate 20/800mg vs. esomeprazole 20mg.
We will describe the relevant methodological characteristics for its understanding:
Design: Randomized, double-blind, active-controlled phase 4 trial. Non-inferiority trial.
Objective: To evaluate the association esomeprazole/sodium bicarbonate vs. standard practice with esomeprazole alone in patients with non-erosive gastroesophageal reflux disease.
Population: Patients with non-erosive gastroesophageal reflux with episodes of heartburn starting at least 3 months before and with a frequency of 2 or more days in the week prior to randomization.
Primary endpoint: Complete resolution of heartburn at 4 weeks of treatment.
Methodology: In the search for the non-inferiority limit, the authors estimated that in previous studies the esomeprazole vs. placebo ratio for complete resolution at 4 weeks was 41 vs. 11%, respectively, that is, a difference of 30% between the groups. To establish the non-inferiority limit, they took 50% of this difference, that is, 15% (0.15) as the limit (this would be the upper limit of the 95% CI).
Results: The proportion of patients without heartburn at 4 weeks was similar in the groups, esomeprazole vs. combined (33.3 vs. 35%, p = 0.737) and the confidence interval for the difference was -0.08 to 0.11, this upper limit is lower than the 0.15 (15%) estimated by the authors, therefore combined medication is considered non-inferior.
In turn, the safety points were evaluated, and adverse events were not different between groups.
Conclusion: The combination of esomeprazole/sodium bicarbonate was not inferior to the non-combined esomeprazole in suppressing heartburn at week 4 of treatment in patients with non-erosive gastroesophageal reflux.
The limiting point of the work is the determination of the non-inferiority limit, which was estimated in relation to previous studies where the difference with placebo of esomeprazole was 30%. This is one of the strengths of the study since references were taken from previously validated studies.
A second consideration is that resolution of heartburn with esomeprazole vs. placebo was 41% and in this study 35%, lower than previously estimated.
If we made the difference between the 35% placebo vs. 11% (24%) and if we estimate the non-inferiority limit with 50% of this difference, this would be 12%, within the 11.39% inferiority limit of the study.
Another point to consider is the safety of the combination vs. the isolated drug, since it is relevant in non-inferiority studies to consider that a new effective drug must also be safe for clinical use, as expressed by the results of this study.
The authors also comment that the combination with sodium bicarbonate would have pharmacodynamic and pharmacokinetic advantages that favor the profile of the drug.
1. In non-inferiority trials it is tested whether the new treatment is non-inferior to the active control.
2. The non-inferiority trials were designed with the objective of evaluating additional advantages of the new treatment with respect to the standard, in important aspects such as: cost, availability, adverse effects, being less invasive and having pharmacological advantages such as pharmacokinetics and pharmacodynamics.
3. The definition of an a priori non-inferiority margin is of essential importance (identifying the source that determined this margin and its confidence intervals) as well as analyzing the results by intention-to-treat or by protocol in this type of trials. Non-inferiority holds only if these two analyses maintain the effect in a coordinated way.
4. The demonstration of non-inferiority does not imply superiority of the new treatment. The superiority trials have as an alternative hypothesis that the new treatment is better than the active treatment or placebo and are analyzed at two tails (p < 0.05), unlike the non-inferiority trial that is analyzed in one tail (p < 0.025).
5. Non-inferiority trials are necessary in situations where it is not ethical to evaluate the new treatment vs. placebo.
Intellectual Property. The author declares that the data and tables that appear in this manuscript are original and were made in his belonging institution.
Funding. The author states that there were no external funding sources.
Conflict of interest. The author declares that he has no conflicts of interest in relation to this article.
Copyright © 2022 Acta Gastroenterológica latinoamericana. This is an
open-access article released under the terms of the Creative Commons Attribution (CC BY-NC-SA 4.0) license,
which allows non-commercial use, distribution, and reproduction, provided the original author and source are
acknowledged.
© 2022 Acta Gastroenterológica latinoamericana. This is an
open-access article released under the terms of the Creative Commons Attribution (CC BY-NC-SA 4.0) license,
which allows non-commercial use, distribution, and reproduction, provided the original author and source are
acknowledged.
Cite this article as: Pizarro Rodolfo. Non-inferiority Clinical Trials. Acta Gastroenterol Latinoam. 2022;52(3):300-302. https://doi.org/10.52787/agl.v52i3.236
Pluchino S. Estudios clínicos de no-inferioridad y de equivalencia: metodología, análisis e interpretación de los resultados Avances Cardiol 2009; 29: 76-81.
Ferreira - González I. Bases para la interpretación de los estudios de no inferioridad: a propósito de los estudios ROCKET-AF, RE-LY y ARISTOTLE. Rev Esp Cardiol 2014;67:432-435.
Ciapponi A. Ensayos aleatorizados de no inferioridad y de equivalencia. Evid Act Pract Ambul 2013;16:92-94.
Leung JT, Barnes SL, Lo ST and Leung DJ. Non-inferiority trials in cardiology: what clinicians need to know. Heart 2020;106:99-104.
Park SH, Lee KN, Lee OY, et al. A Randomized, Double-Blind, Active- Control, Noninferiority, Multicenter, Phase 4 Study to Evaluate the Efficacy and Safety of Esomeprazole/Sodium Bicarbonate 20/800 mg in Patients with Nonerosive Gastroesophageal Reflux Disease. Gut and Liver; June 22, 2022.
Correspondence: Rodolfo Pizarro
Email: rodolfo.pizarro@hospitalitaliano.org.ar
Acta Gastroenterol Latinoam 2022;52(3):300-302