Estudio descriptivo del microambiente inmunológico tumoral e infección por el virus de Epstein-Barr en el adenocarcinoma gástrico
DOI:
https://doi.org/10.52787/agl.v52i4.200Palabras clave:
Neoplasias de estómago, muerte celular programada 1 Ligando 2 Proteína, virus de Epstein-Barr, Herpesvirus 4 Humano, linfocitos T CD8-positivoResumen
Introducción. La región del Cauca, Colombia, tiene una alta incidencia de cáncer gástrico y son pocos los estudios que describen el microambiente tumoral en muestras de cáncer gástrico de esta región geográfica. El objetivo del estudio fue describir la infiltración de células T CD3 + CD8+, la expresión de muerte programada-1 (PD-1) y ligando de muerte programada 1 (PD-L1), y la infección por el virus de Epstein-Barr en biopsias de adenocarcinoma gástrico tipo intestinal avanzado de pacientes de dicha región.
Metodología.
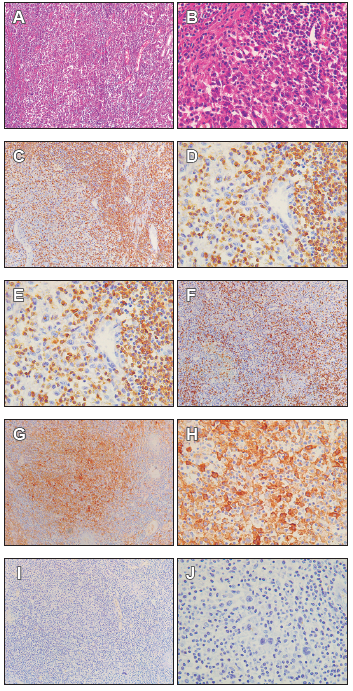
Se trata de un estudio descriptivo transversal realizado en 24 muestras de gastrectomía de las 48 analizadas. La expresión de CD3, CD8, PD-1 y PD-L1 se analizó por inmunohistoquímica y la infección por virus de Epstein-Barr mediante hibridación in-situ de pequeños ARN codificados por el virus de Epstein-Barr (EBER-ISH) en muestras de tejido gástrico fijadas e incluidas en parafina. Finalmente, se calcularon el inmunoscore y el puntaje combinado positivo.
Resultados. Las muestras de adenocarcinoma gástrico tipo intestinal avanzado tenían 21,8 % ± 13,6 % y 14,8 % ± 14,8 % de CD3+ y CD8+ positivo respectivamente, el 100 % tenía baja expresión de PD-1, en puntuación combinado positivo para PDL- 1, el 91,7 % tenía < 1 y el 8,3% tenía > 1. En EBER-ISH el 20,8% dio positivo y en inmunoscore el 16,7% tenía un puntaje > 25%.
Conclusión. Este trabajo reporta casos de adenocarcinoma gástrico tipo intestinal avanzado avanzados con microambiente tumoral atípico, comparado con lo reportado previamente. Es importante considerar que el microambiente tumoral en cáncer gástrico es heterogéneo, por lo que hace relevante el análisis de inestabilidad de microsatélites en este tipo de muestras.
Citas
-1. Liu X, Meltzer SJ. Gastric cancer in the era of precision medicine. Cellular and molecular gastroenterology and hepatology. 2017;3(3):348-58.
-2. Sierra MS, Cueva P, Bravo LE, Forman D. Stomach cancer burden in Central and South America. Cancer epidemiology. 2016;44:S62-S73.
-3. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2021;71(3):209-49.
-4. Ospina ML, Huertas JA, Montaño JI, Rivillas JC. Observatorio nacional de cáncer Colombia. Revista Facultad Nacional de Salud Pública. 2015;33(2):262-76.
-5. Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76(2):182.
-6. Asakawa Y, Okabe A, Fukuyo M, Li W, Ikeda E, Mano Y, et al. Epstein-Barr virus-positive gastric cancer involves enhancer activation through activating transcription factor 3. Cancer science. 2020;111(5):1818.
-7. Sohn BH, Hwang J-E, Jang H-J, Lee H-S, Oh SC, Shim J-J, et al. Clinical significance of four molecular subtypes of gastric cancer identified by the cancer genome atlas project. Clinical Cancer Research. 2017;23(15):4441-9.
-8. Kim TS, Da Silva E, Coit DG, Tang LH. Intratumoral immune response to gastric cancer varies by molecular and histologic subtype. The American journal of surgical pathology. 2019;43(6):851.
-9. Vareki SM, Garrigós C, Duran I. Biomarkers of response to PD-1/PD-L1 inhibition. Critical reviews in oncology/hematology. 2017;116:116-24.
-10. Dabbs DJ. Diagnostic Immunohistochemistry E-Book: Theranostic and Genomic Applications: Elsevier Health Sciences; 2017.
-11. Pagès F, Mlecnik B, Marliot F, Bindea G, Ou F-S, Bifulco C, et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. The Lancet. 2018;391(10135):2128-39.
-12. Yun S, Koh J, Nam SK, Kwak Y, Ahn S-H, Do Park J, et al. Immunoscore is a strong predictor of survival in the prognosis of stage II/III gastric cancer patients following 5-FU-based adjuvant chemotherapy. Cancer Immunology, Immunotherapy. 2021;70(2):431-41.
-13. Mlecnik B, Bifulco C, Bindea G, Marliot F, Lugli A, Lee JJ, et al. Multicenter International Society for Immunotherapy of Cancer study of the consensus Immunoscore for the prediction of survival and response to chemotherapy in stage III colon cancer. J Clin Oncol. 2020:3638-51.
-14. Bruni D, Angell HK, Galon J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nature Reviews Cancer. 2020;20(11):662-80.
-15. Yuan Y, Jiang Y-C, Sun C-K, Chen Q-M. Role of the tumor microenvironment in tumor progression and the clinical applications. Oncology reports. 2016;35(5):2499-515.
-16. Network NCC. National Comprehensive Cancer Network (NCCN) Guidelines. 2015.
-17. Wen T, Wang Z, Li Y, Li Z, Che X, Fan Y, et al. A four-factor immunoscore system that predicts clinical outcome for stage II/III gastric cancer. Cancer immunology research. 2017;5(7):524-34.
-18. Naseem M, Barzi A, Brezden-Masley C, Puccini A, Berger MD, Tokunaga R, et al. Outlooks on Epstein-Barr virus associated gastric cancer. Cancer treatment reviews. 2018;66:15-22.
-19. Zeng D, Zhou R, Yu Y, Luo Y, Zhang J, Sun H, et al. Gene expression profiles for a prognostic immunoscore in gastric cancer. Journal of British Surgery. 2018;105(10):1338-48.
-20. Jiang Y, Zhang Q, Hu Y, Li T, Yu J, Zhao L, et al. ImmunoScore signature: a prognostic and predictive tool in gastric cancer. Annals of surgery. 2018;267(3):504-13.
-21. Jiang Y, Wang H, Wu J, Chen C, Yuan Q, Huang W, et al. Noninvasive imaging evaluation of tumor immune microenvironment to predict outcomes in gastric cancer. Annals of Oncology. 2020;31(6):760-8.
-22. Wang X, Teng F, Kong L, Yu J. PD-L1 expression in human cancers and its association with clinical outcomes. OncoTargets and therapy. 2016;9:5023.
-23. Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD-L1 checkpoint. Immunity. 2018;48(3):434-52.
-24. Xie T, Zhang Z, Zhang X, Qi C, Shen L, Peng Z. Appropriate PDL1 cutoff value for gastric cancer immunotherapy: a systematic review and meta-analysis. Frontiers in oncology. 2021;11:646355.
-25. Yamashita K, Iwatsuki M, Harada K, Eto K, Hiyoshi Y, Ishimoto T, et al. Prognostic impacts of the combined positive score and the tumor proportion score for programmed death ligand-1 expression by double immunohistochemical staining in patients with advanced gastric cancer. Gastric Cancer. 2020;23(1):95-104.
-26. Xing X, Guo J, Ding G, Li B, Dong B, Feng Q, et al. Analysis of PD1, PDL1, PDL2 expression and T cells infiltration in 1014 gastric cancer patients. Oncoimmunology. 2018;7(3):e1356144.
-27. Koh J, Ock C-Y, Kim JW, Nam SK, Kwak Y, Yun S, et al. Clinicopathologic implications of immune classification by PD-L1 expression and CD8-positive tumor-infiltrating lymphocytes in stage II and III gastric cancer patients. Oncotarget. 2017;8(16):26356.
-28. Liang Y, Yu M, Zhou C, Zhu X. Variation of PD-L1 expression in locally advanced cervical cancer following neoadjuvant chemotherapy. Diagnostic Pathology. 2020;15:1-8.
-29. Wang H, Chen X-L, Liu K, Bai D, Zhang W-H, Chen X-Z, et al. Associations between gastric cancer risk and virus infection other than epstein-barr virus: a systematic review and meta-analysis based on epidemiological studies. Clinical and translational gastroenterology. 2020;11(7).
-30. Cho CJ, Kang HJ, Ryu Y-M, Park YS, Jeong HJ, Park Y-M, et al. Poor prognosis in Epstein–Barr virus-negative gastric cancer with lymphoid stroma is associated with immune phenotype. Gastric Cancer. 2018;21(6):925-35.
-31. Gong L-p, Chen J-n, Xiao L, He Q, Feng Z-y, Zhang Z-g, et al. The implication of tumor-infiltrating lymphocytes in Epstein-Barr virus–associated gastric carcinoma. Human pathology. 2019;85:82-91.
-32. Cheng N, Li P, Cheng H, Zhao X, Dong M, Zhang Y, et al. Prognostic Value of Tumor-Infiltrating Lymphocytes and Tertiary Lymphoid Structures in Epstein-Barr Virus-Associated and-Negative Gastric Carcinoma. Frontiers in immunology. 2021;12:2560.
-33. Rodriquenz MG, Roviello G, D’Angelo A, Lavacchi D, Roviello F, Polom K. MSI and EBV positive gastric cancer’s subgroups and their link with novel immunotherapy. Journal of clinical medicine. 2020;9(5):1427.
-34. Ratti M, Lampis A, Hahne JC, Passalacqua R, Valeri N. Microsatellite instability in gastric cancer: molecular bases, clinical perspectives, and new treatment approaches. Cellular and Molecular Life Sciences. 2018;75(22):4151-62.
-35. Derks S, Liao X, Chiaravalli AM, Xu X, Camargo MC, Solcia E, et al. Abundant PD-L1 expression in Epstein-Barr Virus-infected gastric cancers. Oncotarget. 2016;7(22):32925.
-36. Noh B-J, Kim JH, Eom D-W. Prognostic significance of categorizing gastric carcinoma by PD-L1 expression and tumor infiltrating lymphocytes. Annals of Clinical & Laboratory Science. 2018;48(6):695-706.
-37. Pereira MA, Ramos MF, Faraj SF, Dias AR, Yagi OK, Zilberstein B, et al. Clinicopathological and prognostic features of Epstein-Barr virus infection, microsatellite instability, and PDL1 expression in gastric cancer. Journal of surgical oncology. 2018;117(5):829-39.
-38. Sundar R, Qamra A, Tan ALK, Zhang S, Ng CCY, Teh BT, et al. Transcriptional analysis of immune genes in Epstein–Barr virus-associated gastric cancer and association with clinical outcomes. Gastric Cancer. 2018;21(6):1064-70.
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2022 Lucy Bravo-Luna , Rosa Amalia Dueñas-Cuellar, Victoria Eugenia Niño, Angela Merchán-Galvis , Harold Bolaños

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-CompartirIgual 4.0.












