Transaminasas en el primer día poshepatectomía y predicción de resultados en patología maligna
DOI:
https://doi.org/10.52787/agl.v55i4.547Palabras clave:
Hepatectomía, complicaciones postoperatorias, mortalidad, aspartato aminotransferasa, alanina aminotransferasaResumen
Introducción. Los avances en la cirugía hepática oncológica han mejorado la radicalidad de los procedimientos y ampliado los criterios de resecabilidad. Las transaminasas pueden ser indicadores de injuria hepatocelular postoperatoria.
Objetivos. Evaluar la relación entre las transaminasas del primer día postoperatorio y la morbimortalidad en las hepatectomías por patología maligna.
Materiales y métodos. Análisis retrospectivo de hepatectomías por patología maligna entre marzo de 2015 y febrero de 2023. Se evaluaron variables demográficas, intraoperatorias y postoperatorias. Se determinaron los valores de corte óptimos de transaminasas y su relación con las complicaciones y la mortalidad mediante curvas ROC y cálculos de sensibilidad y especificidad.
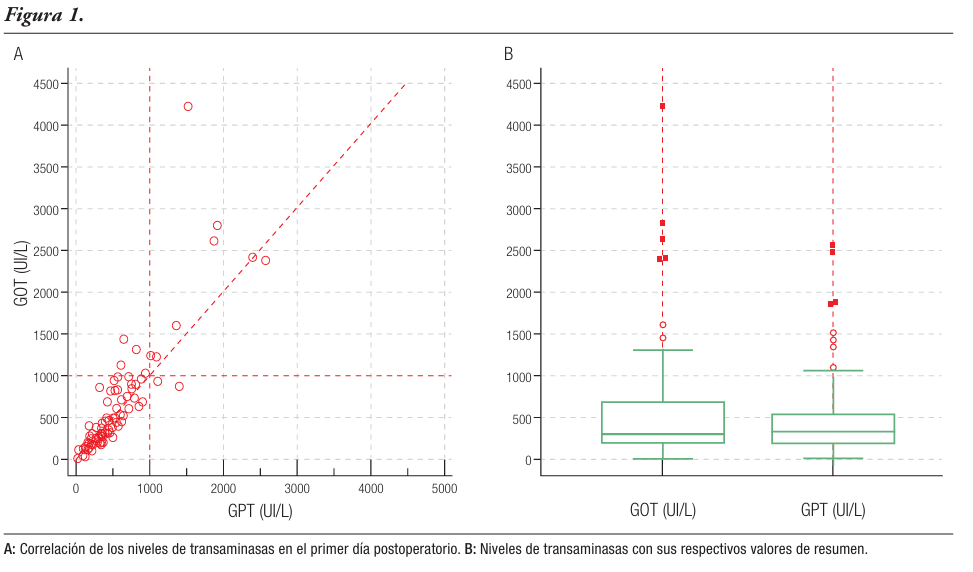
Resultados. Realizamos 273 hepatectomías en nuestra institución, 118 por patología maligna. Hallamos una relación estadísticamente significativa entre los valores elevados de las transaminasas en el primer día postoperatorio y el desarrollo de la morbimortalidad. Los puntos de corte para predecir mortalidad fueron 856 UI/L para la transaminasa glutámico-oxalacética y 1341 UI/L para la transaminasa glutámico-pirúvica (AUC: 0,714 y 0,579, respectivamente), y 530 UI/L y 257 UI/L para predecir la ocurrencia de complicaciones (AUC: 0,700 y 0,663). Los valores de transaminasas glutámico-oxalacética elevados se asociaron a insuficiencia hepática postoperatoria, con un punto de corte > 856 UI/L (AUC: 0,834).
Conclusión. Los valores elevados de transaminasas al primer día postoperatorio se asociaron con mayor ocurrencia de complicaciones, necesidad de hemoderivados y uso de drogas vasoactivas. Una transaminasa glutámico-oxalacética elevada indica un riesgo mayor de insuficiencia hepática postoperatoria y mortalidad a 90 días. Las transaminasas son marcadores pronósticos útiles en las hepatectomías por patología maligna.
Citas
-1. Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, Corvera C, Weber S, Blumgart LH. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236(4):397-407.
-2. Botea F, Ionescu M, Braşoveanu V, Hrehoreţ D, Alexandrescu S, Grigorie M, Stanciulea O, Nicolaescu D, Tomescu D, Droc G, Ungureanu D, Fota R, Croitoru A, Gheorghe L, Gheorghe C, Lupescu I, Grasu M, Boroş M, Dumitru R, Toma M, Herlea V, Popescu I. Liver Resections in a High-Volume Center: From Standard Procedures to Extreme Surgery and Ultrasound-guided Resections. Chirurgia (Bucur). 2017;112(3):259-277.
-3. Dokmak S, Ftériche FS, Borscheid R, Cauchy F, Farges O, Belghiti J. 2012 Liver resections in the 21st century: we are far from zero mortality. HPB (Oxford). 2013;15(11):908-15.
-4. Franken LC, Schreuder AM, Roos E, van Dieren S, Busch OR, Besselink MG, van Gulik TM. Morbidity and mortality after major liver resection in patients with perihilar cholangiocarcinoma: A systematic review and meta-analysis. Surgery. 2019;165(5):918-928.
-5. Azoulay D, Bhangui P, Pascal G, Salloum C, Andreani P, Ichai P, Saliba F, Lim C. The impact of expanded indications on short-term outcomes for resection of malignant tumours of the liver over a 30 year period. HPB (Oxford). 2017;19(7):638-648.
-6. Mahlmann JC, Wirth TC, Hartleben B, Schrem H, Mahlmann JF, Kaltenborn A, Klempnauer J, Kulik U. Chemotherapy and Hepatic Steatosis: Impact on Postoperative Morbidity and Survival after Liver Resection for Colorectal Liver Metastases. Visc Med. 2021;37(3):198-205.
-7. Bagante F, Ruzzenente A, Beal EW, Campagnaro T, Merath K, Conci S, Akgül O, Alexandrescu S, Marques HP, Lam V, Shen F, Poultsides GA, Soubrane O, Martel G, Iacono C, Guglielmi A, Pawlik TM. Complications after liver surgery: a benchmark analysis. HPB (Oxford). 2019;21(9):1139-1149.
-8. Rössler F, Sapisochin G, Song G, Lin YH, Simpson MA, Hasegawa K, Laurenzi A, Sánchez Cabús S, Nunez MI, Gatti A, Beltrame MC, Slankamenac K, Greig PD, Lee SG, Chen CL, Grant DR, Pomfret EA, Kokudo N, Cherqui D, Olthoff KM, Shaked A, García-Valdecasas JC, Lerut J, Troisi RI, De Santibanes M, Petrowsky H, Puhan MA, Clavien PA. Defining Benchmarks for Major Liver Surgery: A multicenter Analysis of 5202 Living Liver Donors. Ann Surg. 2016;264(3):492-500.
-9. Aloia TA, Fahy BN, Fischer CP, Jones SL, Duchini A, Galati J, Gaber AO, Ghobrial RM, Bass BL. Predicting poor outcome following hepatectomy: analysis of 2313 hepatectomies in the NSQIP database. HPB (Oxford). 2009;11(6):510-5.
-10. Vasavada B, Patel K. Postoperative Morbidity after Liver Resection - A Systemic Review, Meta-analysis and Metaregression of Factors Affecting Them. Surg Chron. 2022;27(2):143-149.
-11. Boleslawski E, Vibert E, Pruvot FR, Le Treut YP, Scatton O, Laurent C, Mabrut JY, Régimbeau JM, Adham M, Cosse C, Farges O. Relevance of postoperative peak transaminase after elective hepatectomy. Ann Surg. 2014;260(5):815-20; discussion 820-1.
-12. Bhogal RH, Nair A, Papis D, Hamady Z, Ahmad J, Lam FT, Khan S, Marangoni G. Postoperative day one serum alanine aminotransferase does not predict patient morbidity and mortality after elective liver resection in non-cirrhotic patients. Hepatobiliary Pancreat Dis Int. 2016;15(6):655-659.
-13. Grąt M, Hołówko W, Lewandowski Z, Kornasiewicz O, Barski K, Skalski M, Zieniewicz K, Krawczyk M. Early post-operative prediction of morbidity and mortality after a major liver resection for colorectal metastases. HPB (Oxford). 2013;15(5):352-8.
-14. de Klein GW, Brohet RM, Liem MSL, Klaase JM. Post-operative Day 1 Serum Transaminase Levels in Relation to Morbidity After Liver Resection. World J Surg. 2022;46(2):433-440.
-15. Olthof PB, Huiskens J, Schulte NR, Wicherts DA, Besselink MG, Busch OR, Heger M, van Gulik TM. Postoperative peak transaminases correlate with morbidity and mortality after liver resection. HPB (Oxford). 2016;18(11):915-921.
-16. D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44(1):217-31.
-17. Lee JS, Choi HW, Kim JS, Lee TY, Yoon YC. Update on Resection Strategies for Hepatocellular Carcinoma: A Narrative Review. Cancers (Basel). 2024;16(23):4093.
-18. Delis SG, Bakoyiannis A, Biliatis I, Athanassiou K, Tassopoulos N, Dervenis C. Model for end-stage liver disease (MELD) score, as a prognostic factor for post-operative morbidity and mortality in cirrhotic patients, undergoing hepatectomy for hepatocellular carcinoma. HPB (Oxford). 2009;1(4):351-7.
-19. Elshaarawy O, Aman A, Zakaria HM, Zakareya T, Gomaa A, Elshimi E, Abdelsameea E. Outcomes of curative liver resection for hepatocellular carcinoma in patients with cirrhosis. World J Gastrointest Oncol. 2021;13(5):424-439.
-20. Torzilli G, Viganò L, Cimino M, Imai K, Vibert E, Donadon M, Mansour D, Castaing D, Adam R. Is Enhanced One-Stage Hepatectomy a Safe and Feasible Alternative to the Two-Stage Hepatectomy in the Setting of Multiple Bilobar Colorectal Liver Metastases? A Comparative Analysis between Two Pioneering Centers. Dig Surg. 2018;35(4):323-332.
-21. Torzilli G, Serenari M, Viganò L, Cimino M, Benini C, Massani M, Ettorre GM, Cescon M, Ferrero A, Cillo U, Aldrighetti L, Jovine E. Outcomes of enhanced one-stage ultrasound-guided hepatectomy for bilobar colorectal liver metastases compared to those of ALPPS: a multicenter case-match analysis. HPB (Oxford). 2019;21(10):1411-1418.
-22. Ironside N, Bell R, Bartlett A, McCall J, Powell J, Pandanaboyana S. Systematic review of perioperative and survival outcomes of liver resections with and without preoperative portal vein embolization for colorectal metastases. HPB (Oxford). 2017;19(7):559-566.
-23. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-83.
-24. Pang YY. The Brisbane 2000 terminology of liver anatomy and resections. HPB. 2000;2:333-39. HPB (Oxford). 2002;4(2):99; author reply 99-100.
-25. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205-213.
-26. Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo RP, Christophi C, Banting S, Usatoff V, Nagino M, Maddern G, Hugh TJ, Vauthey JN, Greig P, Rees M, Yokoyama Y, Fan ST, Nimura Y, Figueras J, Capussotti L, Büchler MW, Weitz J. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149(5):713-24.
-27. Sultana A, Brooke-Smith M, Ullah S, Figueras J, Rees M, Vauthey JN, Conrad C, Hugh TJ, Garden OJ, Fan ST, Crawford M, Makuuchi M, Yokoyama Y, Büchler M, Padbury R. Prospective evaluation of the International Study Group for Liver Surgery definition of post hepatectomy liver failure after liver resection: an international multicentre study. HPB (Oxford). 2018;20(5):462-469.
-28. Mullen JT, Ribero D, Reddy SK, Donadon M, Zorzi D, Gautam S, Abdalla EK, Curley SA, Capussotti L, Clary BM, Vauthey JN. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204(5):854-62; discussion 862-4.
-29. Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, Durand F. The "50-50 criteria" on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242(6):824-8, discussion 828-9.
-30. Mayo SC, Shore AD, Nathan H, Edil BH, Hirose K, Anders RA, Wolfgang CL, Schulick RD, Choti MA, Pawlik TM. Refining the definition of perioperative mortality following hepatectomy using death within 90 days as the standard criterion. HPB (Oxford). 2011;13(7):473-82.
-31. Egger ME, Ohlendorf JM, Scoggins CR, McMasters KM, Martin RC 2nd. Assessment of the reporting of quality and outcome measures in hepatic resections: a call for 90-day reporting in all hepatectomy series. HPB (Oxford). 2015;17(9):839-45.
-32. Schiergens TS, Dörsch M, Mittermeier L, Brand K, Küchenhoff H, Lee SM, Feng H, Jauch KW, Werner J, Thasler WE. Thirty-day mortality leads to underestimation of postoperative death after liver resection: A novel method to define the acute postoperative period. Surgery. 2015;158(6):1530-7.
-33. Azoulay D, Pascal G, Salloum C, Adam R, Castaing D, Tranecol N. Vascular reconstruction combined with liver resection for malignant tumours. Br J Surg. 2013;100(13):1764-75.
-34. Giovannini I, Chiarla C, Giuliante F, Vellone M, Ardito F, Sarno G, Nuzzo G. Analysis of the components of hypertransaminasemia after liver resection. Clin Chem Lab Med. 2007;45(3):357-60.
-35. Gurusamy KS, Sheth H, Kumar Y, Sharma D, Davidson BR. Methods of vascular occlusion for elective liver resections. Cochrane Database Syst Rev. 2009;Jan 21;(1):CD007632.
-36. Belghiti J, Noun R, Malafosse R, Jagot P, Sauvanet A, Pierangeli F, Marty J, Farges O. Continuous versus intermittent portal triad clamping for liver resection: a controlled study. Ann Surg. 1999;229(3):369-75.
-37. van den Broek MA, Shiri-Sverdlov R, Schreurs JJ, Bloemen JG, Bieghs V, Rensen SS, Dejong CH, Olde Damink SW. Liver manipulation during liver surgery in humans is associated with hepatocellular damage and hepatic inflammation. Liver Int. 2013;33(4):633-41.
-38. van de Poll MC, Derikx JP, Buurman WA, Peters WH, Roelofs HM, Wigmore SJ, Dejong CH. Liver manipulation causes hepatocyte injury and precedes systemic inflammation in patients undergoing liver resection. World J Surg. 2007;31(10):2033-8.
-39. Fung J, Poon RT, Yu WC, Chan SC, Chan AC, Chok KS, Cheung TT, Seto WK, Lo CM, Lai CL, Yuen MF. Use of liver stiffness measurement for liver resection surgery: correlation with indocyanine green clearance testing and post-operative outcome. PLoS One. 2013;8(8):e72306.
-40. Vassanasiri W, Rungsakulkij N, Suragul W, Tangtawee P, Muangkaew P, Mingphruedhi S, Aeesoa S. Early postoperative serum aspartate aminotransferase for prediction of post-hepatectomy liver failure. Perioper Med (Lond). 2022;11(1):51.
-41. Boissel JP, Collet JP, Moleur P, Haugh M. Surrogate endpoints: a basis for a rational approach. Eur J Clin Pharmacol. 1992;43(3):235-44.
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2025 Lourdes Mollard, Rodrigo A Gasque, Magalí Chahdi Beltrame, Marcelo E Lenz, Francisco J Mattera, Emilio G Quiñonez

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-CompartirIgual 4.0.












