Argentina and Chile Data Analysis from the ICONIC Observational Study
DOI:
https://doi.org/10.52787/agl.v52i4.233Keywords:
Ulcerative colitis, suffering, patient-reported outcomes, PRISMAbstract
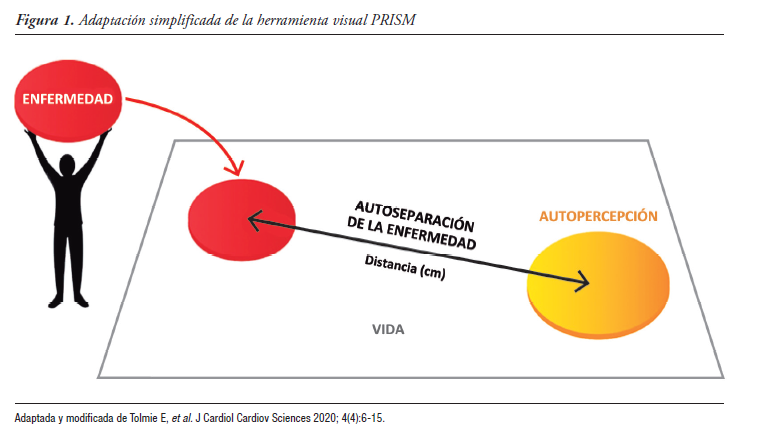
Introduction. The prospective observational ICONIC study assessed, using the Pictorial Representation of Illness and Self Measure (PRISM) tool, the burden of suffering caused to patients by ulcerative colitis (UC).
Methods. ICONIC included outpatients with recent-onset UC in 33 countries. Patient- and physician-reported outcomes (PRISM, Short Inflammatory Bowel Disease Questionnaire [SIBDQ], Patient Health Questionnaire [PHQ-9], Simple Clinical Colitis Activity Indexes [P-SCCAI; SCCAI]) were collected at baseline and every 6 ± 3 months. Data from Argentina and Chile are described in this analysis.
Results. 89 patients (age: median 32, range: 18-63; 52.8% female; left UC: 50%; proctitis: 33.3%; extensive UC: 16.7%) had ≥ 1 follow-up visit. The mean ± SD of severity (SCCAI/pSCCAI) significantly decreased from 1.8 ± 2.2 to 1.4 ± 2.0 (p < 0.05) and from 3.3 ± 3.2 to 2.6 ± 2.8, respectively, over 24 months. Suffering assessed by patients and physicians, progressed to extensive UC. Patient- and physician-rated suffering, as quantified by PRISM, was significantly reduced, showing a correlation with improvement in other outcome measures. An underestimation of physician-assessed suffering by PRISM compared to patient data was described.
Conclusions. The burden and suffering decreased with
relatively early treatment. Consistent with the overall study, PRISM was correlated with other measures of UC perception; suffering was underestimated by physicians. PRISM, given its correlation with other scales, it may be considered a useful tool to quantify distress in patients with UC.
References
-1. Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389(10080):1756-70.
-2. McCormick JB, Hammer RR, Farrell RM, Geller G, James KM, Loftus Jr EV, Mercer MB, Tilburt JC, Sharp RR. Experiences of patients with chronic gastrointestinal conditions: in their own words. Health Qual Life Outcomes. 2012;10:25.
-3. Yarlas A, Rubin DT, Panés J, Lindsay JO, Vermeire S, Bayliss M, Cappelleri JC, Maher S, Bushmakin AG, Chen LA, DiBonaventura M. Burden of Ulcerative Colitis on Functioning and Well-being: A Systematic Literature Review of the SF-36® Health Survey. J Crohns Colitis. 2018;12(5):600-9.
-4. Schreiber S, Panés J, Louis E, Holley D, Buch M, Paridaens K. Perception gaps between patients with ulcerative colitis and healthcare professionals: an online survey. BMC Gastroenterol. 2012;12:108.
-5. Cassel EJ. The nature of suffering and the goals of medicine. N Engl J Med. 1982;306:639-45.
-6. Ghosh S, Sensky T, Casellas F, Rioux LC, Ahmad T, Márquez JR, Vanasek T, Gubonina I, Sezgin O, Ardizzone S, Kligys K, Petersson J, Suzuki Y, Peyrin-Biroulet L. A global, prospective, observational study measuring disease burden and suffering in patients with ulcerative colitis using the Pictorial Representation of Illness and Self Measure Tool. J Crohns Colitis. 2020;15(2):228-37.
-7. Büchi S, Sensky T. PRISM: Pictorial Representation of Illness and Self Measure. A brief nonverbal measure of illness impact and therapeutic aid in psychosomatic medicine. Psychosomatics. 1999;40(4):314-20.
-8. Peter N, Kleinjung T, Horat L, Schmidt-Weitmann S, Meyer M, Büchi S, Weidt S. Validation of PRISM (pictorial representation of illness and self measure) as a novel visual assessment tool for the burden of suffering in tinnitus patients. Health Qual Life Outcomes. 2016;14:47.
-9. Kabar I, Hüsing-Kabar A, Maschmeier M, Völler C, Dümke M, Schmidt HH, Heinzow H. Pictorial representation of illness and self measure (PRISM): a novel visual instrument to quantify suffering in liver cirrhosis patients and liver transplant recipients. Ann Transplant. 2018;23:674-80.
-10. Krikorian A, Limonero JT, Vargas JJ, Palacio C. Assessing suffering in advanced cancer patients using pictorial representation of illness and self measure (PRISM), preliminary validation of the Spanish version in a Latin American population. Support Care Cancer. 2013;21:3327-36.
-11. Rubin DT, Dubinsky MC, Martino S, Hewett KA, Panés J. Communication between physicians and patients with ulcerative colitis: reflections and insights from a qualitative study of in-office patient-physician visits. Inflamm Bowel Dis. 2017;23:494-501.
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Alicia Sambuelli, Gustavo Walsen Arangua, Juan Andrés de Paula, Juan José Trakal, Martín Toro, Martín Yantorno, Soledad Suárez Ordóñez

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.












