Hepatocellular Carcinoma: Epidemiological Reality in Tucumán, Argentine Northwest
DOI:
https://doi.org/10.52787/agl.v53i3.337Keywords:
Hepatocellular carcinoma, epidemiology, cirrhosisAbstract
Introduction. Hepatocellular carcinoma is the most common primary liver tumor (80 to 90%). It is the sixth most frequent tumor and the third that causes the most cancer-related deaths. 85% occurs in Asia and Africa. In Latin America and Argentina, it is not fully represented in the literature due to the socio-economic reality. Most hepatocellular carcinomas present on the basis of cirrhosis.
Objective. To characterize the epidemiology of hepatocellular carcinoma in Tucumán, Argentina.
Material and Method. A descriptive, multicentric, study of both public and private activity in the province was carried out. Based on international guidelines, diagnosis and treatment of patients with hepatocellular carcinoma were carried out.
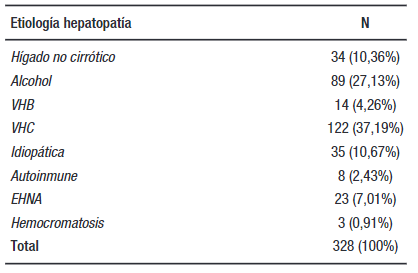
Results. There were 328 patients, with 109 (33.23%) women and 219 (66.76%) men. Of these patients, 294 (89.6%) had cirrhotic liver. The 5-year survival was 6.09% (n:20/328) in stage A and 0.914% (n:3/328) in stage B.
Conclusion. The epidemiology of hepatocellular carcinoma in Tucumán, Argentina was updated; coinciding with the literature. From the epidemiological point of view it showed; a higher prevalence of the disease in males than in females; that stage D at the time of diagnosis was the most prevalent; and that the majority of patients suffered from previous liver diseases.
References
-1. International Agency for Research and Cancer. World Health Organization. Disponible en https://gco.iarc.fr/today/data/fact-sheets/populations/900-world-fact-sheets.pdf; consultado en septiembre de 2021.
-2. Petrick J L, Florio AA, Znaor A, et al. International trends in hepatocellular carcinoma incidence, 1978-2012. Int J Cancer. 2020; 147: 317-30.
-3. Ratziu V, Bellentani S, Cortez-Pinto H, et al. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. 2010;53:372-384.
-4. Asociación Argentina para el Estudio de las Enfermedades del Hígado. Guía de práctica clínica sobre la prevención, vigilancia, diagnóstico, estadificación y tratamiento del hepatocarcinoma. Disponible en: https://www. sahe.org.ar/es/attachment/show/45; consultado en febrero de 2022.
-5. European Association For The Study of the Liver; European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol. 2012;56:908-43.
-6. Federico Piñero, Jaime Poniachik, Ezequiel Ridruejo, Marcelo Silva. Hepatocellular carcinoma in Latin America: Diagnosis and treatment challenges World J Gastroenterol. 2018;24 (37):4224-9.
-7. Federico Piñero et al. Adherence to Barcelona Clinic Liver Cancer therapeutic algorithm for hepatocellular carcinoma in the daily practice: a multicenter cohort study from Argentina. European Journal of Gastroenterology & Hepatology. 2018;30:376-83.
-8. Debes Jose, Aaron J. Chan, Domingo Balderramo, Luciana Kikuchi, Esteban Gonzalez Ballerga, Jhon E. Prieto, et al. Hepatocellular carcinoma in South America: Evaluation of risk factors, demographics and therapy. Liver Int. 2018;38(1):136-43.
-9. Objetivos, metas y estrategias sanitarias 2007-2016 “Salud al Bicentenario”. Capítulo 4, página 124. Provincia de Tucumán. República Argentina.
-10. Clarinda W. Chua and Su Pin Choo. L, et al. Targeted Therapy in Hepatocellular Carcinoma. International Journal of Hepatology. 2011. Article ID 348297:10.4061/2011/34829
-11. Sho T, Suda G, Ogawa K, et al. Early response and safety of atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma in patients who do not meet IMbrave150 eligibility criteria. Hepatol Res. 2021;51:979-89.
-12. Iwamoto H, Shimose S, Noda Y, et al. Initial experience of atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma in real-world clinical practice. Cancers (Basel) 2021;13:2786.
-13. Hiraoka A, Kumada T, Tada T, et al. Atezolizumab plus bevacizumab treatment for unresectable hepatocellular carcinoma: early clinical experience. Cancer Rep (Hobo- ken). 2022;5:e1464.
-14. Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. EASL Panel of Experts on HCC. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-30.
-15. European Association for The Study of the Liver; European Organisation for Research and Treatment of Cancer. EASL-EO-RTC clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol. 2012;56:908-43.
-16. Forner A y colaboradores. Diagnóstico y tratamiento del carcinoma hepatocelular. Actualización del documento de consenso de la AEEH, SEOM, SERAM, SERVEI y SETH. Med Clin (Barc). 2016. http://dx.doi.org/10.1016/j.medcli.2016.01.028
-17. Freddie Bray, et al. Global Cancer Statistics 2018: GLOBOCAN. Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer Journal for Clinicians. 2018;0:1-31.
-18. Peter R. Galle, Alejandro Forner, et al. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. Journal of Hepatology. 2018; 69:182-236.
-19. Liu Y, Li H, Ye N, et al. Non-cirrhotic liver is associated with poor prognosis of hepatocellular carcinoma: a literature review. Med Sci Monit. 2019;25:6615-23.
-20. Wörns MA, Bosslet T, Victor A, et al. Prognostic factors and out-comes of patients with hepatocellular carcinoma in non-cirrhotic liver. Scand J Gastroenterol. 2012;47:718-28.
-21. Smoot RL, Nagorney DM, Chandan VS, et al. Resection of hepatocellular carcinoma in patients without cirrhosis. Br J Surg. 2011;98:697-703.
-22. Giannini EG, Marenco S, Bruzzone L, et al. Hepatocellular carcinoma in patients without cirrhosis in Italy. Dig Liver Dis. 2013;45:164-9.
-23. International Agency for Research on Cancer. Cancer Today. Disponible en: http://gco.iarc.fr/today/home; consultado en mayo de 2021.
-24. Dirchwolf M, Marciano S , Ruf AE, Singal AG, D’Ercole V, et al. Failure in all steps of hepatocellular carcinoma surveillance process is frequent in daily practice. Annals of Hepatology. 2021;25:100344.
-25. Shreya Sengupta, Neehar D Parikh. Biomarker development for hepatocellular carcinoma early detection: current and future perspectives. Hepat. Oncol. 2017; 4(4):111-22.
-26. Yukihiro Haruyama, Hiroaki Kataoka. Glypican-3 is a prognostic factor and an immunotherapeutic target in hepatocellular carcinoma. World J Gastroenterol. 2016;22(1):275-83.
-27. Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018. DOI: 10.1038/s41571-018-0073-4
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Fernando Buabse, Felipe Palazzo, Ana Palazzo, Dolores Murga, Daniela Perez, Moira Zunino, Claudia Gadea, Carlos Garrocho, Juan José Rodríguez, Rodrigo Segovia, Analía Soria, Pablo Berarducchi, Iris Aybar, Marcela Ortiz Mayor, Carmen Seoane, Nancy Soria, Daniela Lionetti, Marcelo López Avellaneda, Germán Alanís, Marcelo Ferraro, Santiago Villavicencio, Roxana González

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.












